Can we prevent neurons from being eaten alive after injury to the motor system?
Imagine that you want to type notes during class, reach for your cup of coffee, or practice the violin. These actions are governed by the central nervous system, which is made up of our brain and our spinal cord. The motor network that makes these activities possible originates in the brain and ultimately results in the execution of movement through spinal motor systems. Brain areas such as the motor cortex, basal ganglia, and cerebellum are critical for planning and initiating movements, such as when we ride a bike. However, when we as humans button our jackets or perform movements involving fine motor skills, we utilize our corticospinal system, which is essential for skilled and voluntary movements in humans and many animals.
The corticospinal system is comprised of the motor cortex and the corticospinal tract. The corticospinal tract is the major descending pathway for motor control of voluntary movements with descending axons, the long “highways” of our brain cells, that originate from the primary motor cortex (on top of our brain) and terminate in the cervical spinal cord (by our neck).
After spinal cord injury, damage to the corticospinal tract often leads to the loss of upper limb and hand function, especially after cervical injury. This means that key functions that involve voluntary movement become difficult to perform because neurons in the corticospinal tract cannot relay the brain’s desired motor command to the spinal cord to execute movements. However, microglia, the resident myeloid cells in the central nervous system, also play a key role in spinal cord injury. These cells are involved in the body’s innate immune response and are needed to keep neurons healthy. They do so by phagocytosis, where they “eat up” myelin debris after spinal cord injury. However, sometimes their activation can be detrimental to the health of neurons, like when they phagocytose neurons that are alive.
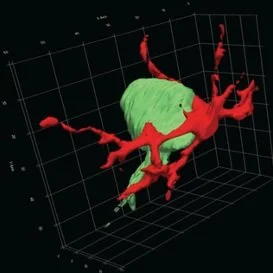
In a rat model of neuronal degeneration, this 3D reconstruction, from a series of microscopy images, shows a microglial cell (red) contacting a neuron (green) in the spinal cord. (Jiang, Sarkar et al. 2018).
Recent work published in The Journal of Neuroscience demonstrated that corticospinal tract injury drives transneuronal degeneration of the spinal motor circuit (Jiang, Sarkar et al. 2018). This means that after injury to the brain, neurons begin to degenerate in areas that are downstream from the injury site, like in the cervical spinal cord. In a rat model, the researchers found that during the first week of injury, microglial activation preceded neuronal death and contributed to transneuronal degeneration by phagocytosing neurons that were still alive, or “non-apoptotic”. The researchers asked how this mix-up happened and found that there was innate immune molecule deposits on non-apoptotic neurons before their phagocytosis. This indicated that the neurons were marked by the innate immune molecules for microglia phagocytosis although the neurons were non-apoptotic.
Is there a way to save the non-apoptotic neurons from this fate?
Intriguingly, this loss of non-apoptotic premotor interneurons can be ameliorated by a form of neuromodulation (alternating nerve activity, using electrical stimulation, at specific sites on the body) called “trans-spinal direct current stimulation” or tsDCS. tsDCS generates a high-density electric field along the spinal cord where the stimulating electrodes are placed, and mathematical modeling can be used to position the stimulating electrodes to maximize current density to specific regions. These dense electric fields can then activate the neurons that would once be active had the injury not occurred.
tsDCS is well-suited for motor rehabilitation because it is a non-invasive therapy. This therapy has been shown to prevent transneuronal degeneration when the electrodes are placed on the cervical enlargement, where we would otherwise find degeneration of non-apoptotic cells. This suggests that maintaining neural activity through an external source can reduce neuron loss and maintain the integrity of the spinal cord motor circuit. There is much more research that needs to be done, but this is an important first step towards improving motor behavior after corticospinal tract injury.
Jiang, Y. Q., A. Sarkar, A. Amer and J. H. Martin (2018). "Transneuronal Downregulation of the Premotor Cholinergic System After Corticospinal Tract Loss." J Neurosci 38(39): 8329-8344.
Jasmine Pathan is a Neuroscience PhD candidate at the City University of New York (CUNY) Graduate Center in NYC. Her passion for understanding the brain compelled her to study neuroscience—the nexus between biology and psychology. Jasmine spent most of her childhood in the concrete jungle of NYC before moving to a rural village in Taiwan, then moving back to New York to finish high school. She ventured across the country to the West Coast for college, graduating with a Bachelor of Arts in Psychology from the University of Washington in 2018. Jasmine’s doctoral research focuses on how different methods of neuromodulation can prevent transneuronal degeneration of key spinal neuron classes while modulating the innate immune response after a spinal cord injury. Outside of being a scientist, Jasmine also likes doing Pilates to stay active, reading non-fiction books, and mastering pottery to make planters for her many plants! Jasmine is also the neuroscience student representative to the Biology PhD Program Executive Committee at CUNY where she advocates for student well-being.
Edited by Denise Croote, PhD